
Note: This story was updated at 5:30 p.m.
The Y-12 National Security Complex in Oak Ridge is supporting a program to make an isotope used in more than 40,000 medical procedures across the nation each day. The goal is to produce the isotope, molybdenum-99 (Mo-99), in the United States without using highly enriched uranium.
Some of that work could occur in Oak Ridge. A company called CoquĂ Radio Pharmaceuticals Corporation announced in April that it plans to build a $500 million medical isotope production facility at the Heritage Center in west Oak Ridge. CoquĂ’s facility would make medical isotopes, primarily Mo-99, and the company could start production in 2025.
In a video posted online, Y-12 said some of its researchers have extensive knowledge of Mo-99 and are sharing that information with CoquĂ and other companies hoping to produce the isotope.
Y-12 has been involved with Mo-99 since 2009, said Cole Jackson of Y-12 Global Security and Strategic Partnerships.
“We’ve been working on it for some time now,” Jackson said.
He said Y-12 is the expert in uranium fabrication, and the National Nuclear Security Administration, which oversees work at Y-12, has made the technical expertise open and available to the companies interested in producing Mo-99. The industry partnerships involve working with companies that are willing to use low enriched uranium or non-highly enriched uranium to make Mo-99, Jackson said.
All of the isotope used now is supplied by foreign companies, and most of them use highly enriched uranium to make the isotope, the NNSA said in a story posted on its website.
The NNSA, which is part of the U.S. Department of Energy, is leading the Mo-99 program and working with several companies to produce a redundant, reliable supply of the isotope in the United States. It’s part of the agency’s mission to reduce the use of highly enriched uranium, or HEU.
The program requires cooperation and coordination among government, industry, and the medical community, the NNSA said.
“The ultimate goal of the Mo-99 program is to ensure that this important medical isotope is readily available to meet patient needs, and is produced in accordance with U.S. nuclear nonproliferation policy by eliminating the use of proliferation-sensitive HEU,” the NNSA said.
In the Y-12 video, Carmen Bigles, founder and chief executive officer of CoquĂ, said there are a half-dozen reactors around the world producing Mo-99, and two of them are set to shut down in the next decade, one in 2024 and the other in 2025.
So, it’s critical that the CoquĂ facility be up and running by the end of 2025, Bigles said. It’s strategically important that the planned facility in Oak Ridge is close to Y-12 and Oak Ridge National Laboratory, she said in April.
The NNSA said Mo-99 is mostly produced in aging research reactors that have experienced more unplanned outages in recent years.
“Some of these outages have caused global Mo-99 supply shortages, in some cases forcing hospitals to delay critical patient treatment,” the NNSA said.
The agency said it has worked since 2009 to accelerate the commercial production of Mo-99 in the United States by supporting a variety of innovative technologies that do not use highly enriched uranium. It seeks to create a redundant, reliable commercial supply, the NNSA said.
The mission was made more urgent by the shutdown of Canada’s National Research Universal reactor—historically the United States largest supplier—in late 2016, the NNSA said.
To support the development of production in the United States, NNSA agreements in 2010 provided up to $25 million in matching funds for the program, based on a 50/50 cost-share between the NNSA and commercial partners in the U.S.
The NNSA currently has initial cooperative agreements with three partners for four projects: Northstar Medical Radioisotopes (two projects), SHINE Medical Technologies, and General Atomics.
The NNSA said it is staying neutral on the technology, recognizing that a successful solution to preventing an Mo-99 shortage is more likely to be found if several production technologies are developed at the same time.
Here is some information about the technologies being developed:
- NorthStar Medical Radioisotopes is developing both neutron capture and accelerator-based technologies.
- SHINE Medical Technologies is developing accelerator technology to produce Mo-99 with accelerator-based low enriched uranium fission.
- General Atomics is working with the Missouri University Research Reactor and Nordion to produce fission-based Mo-99 from a low enriched uranium target using selective gas extraction technology.
In 2018, NNSA issued a new funding opportunity announcement for the production of Mo-99 without the use of highly enriched uranium. In February 2019, Energy Secretary Rick Perry announced that four U.S. companies would begin negotiations for potential new cooperative agreement awards:
- NorthStar Medical Radioisotopes LLC, located in Beloit, Wisconsin
- SHINE Medical Technologies, located in Janesville, Wisconsin Northwest
- Medical Isotopes, located in Corvallis, Oregon
- Niowave Inc., located in Lansing, Michigan
On Monday, the NNSA announced that it has awarded three agreements, based upon the evaluations and recommendations of an independent panel. The companies that will receive a cooperative agreement award are:
- Niowave Inc.
- NorthStar Medical Radioisotopes LLC
- SHINE Medical Technologies
Negotiations continue for a fourth cooperative agreement with Northwest Medical Isotopes, the NNSA said.
Congress appropriated $40 million for the awards in fiscal year 2018 and $20 million in fiscal year 2019 and directed DOE to issue a funding opportunity announcement to competitively award the cooperative agreements, the press release said. NNSA will fund each agreement at $15 million and require each awardee to provide $15 million of matching funds.
“Mo-99 is a critical medical isotope that empowers us to fight back against heart disease and cancer,” said Lisa E. Gordon-Hagerty, DOE under secretary for nuclear security and NNSA administrator. “These agreements will facilitate its domestic production without highly enriched uranium, greatly reducing the potential for proliferation of nuclear materials.”
Besides the agreements, the NNSA also makes technical expertise from the national laboratories available to existing and potential Mo-99 producers who want help in converting their Mo-99 production processes to use low enriched uranium targets or to develop Mo-99 production technologies that don’t use highly enriched uranium, or HEU.
NNSA said it also funds national laboratories to advance industry efforts to produce Mo-99 domestically without HEU. In 2019, NNSA is funding national lab work in support of NorthStar, SHINE, Northwest Medical Isotopes, Niowave, as well as BWXT Isotope Technology Group, CoquĂ Radio Pharmaceuticals, Global Medical Isotope Systems, and Magneto Inertial Fusion Technologies Inc.
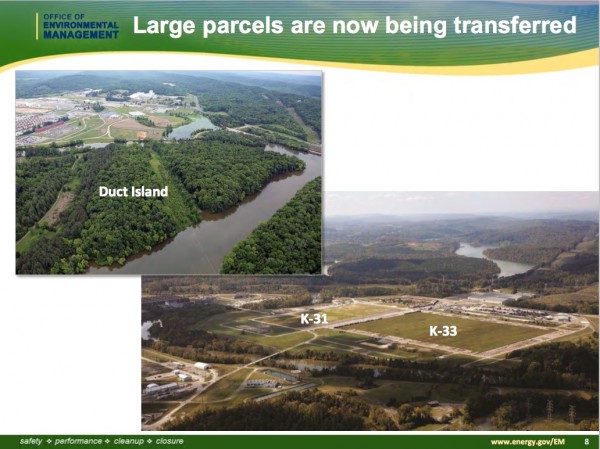
In April, CoquĂ said the U.S. Department of Energy had transferred land for its Oak Ridge facility and provided research support through the national laboratories. The Heritage Center site, known as Duct Island, was transferred to CoquĂ in late September. Heritage Center is the former K-25 site.
The project will need to be licensed by the U.S. Nuclear Regulatory Commission. In April, CoquĂ said it would prepare an environmental report and could apply for an NRC construction permit in mid-2020.
Once it begins operating, the CoquĂ facility will be the first of its kind in the United States, the company said.
“CoquĂ is best positioned to meet the demand for lifesaving medical isotopes because our technology is commercially proven and is used in the current supply chain,” Bigles said in the April press release. The design of CoquĂ’s facility is being led by INVAP, the world’s leading designer and developer of medical isotope production facilities, the release said. CoquĂ has an exclusive license with INVAP to use its technology in the United States.
Besides the land transfer, the press release said DOE has provided support for the CoquĂ facility through research funding. Those funds support CoquĂ’s partnership with ORNL and Y-12 to conduct further research on Mo-99 target plate fabrication and qualification. CoquĂ’s decision to locate its production facility in Oak Ridge makes its collaboration with ORNL and Y-12 easier and more efficient, the press release said.
“This research partnership is critical and supports our efforts to obtain Nuclear Regulatory Commission and Food and Drug Administration approvals for the facility,” Bigles said in the press release. “The sooner we can begin producing U.S.-made Mo-99, the sooner we can minimize our dependency on foreign imports to meet critical U.S. medical needs.”
Officials advocating for the proposed Oak Ridge Airport at the Heritage Center have cited CoquĂ and its need to fly out short-lived medical isotopes as one reason to build the airport in west Oak Ridge.
The decay product of Mo-99, technetium-99m (Tc-99m), is used to diagnose heart disease and cancer, study organ structure and function, and perform other important medical applications. Patients undergoing a common procedure—the cardiac stress test—have probably benefited from Tc-99m, the NNSA said.
The press release said the United States is supporting companies to achieve the objective of supplying approximately 3,000 six-day curies of Mo-99 per week. The American Medical Isotopes Production Act of 2012 directed NNSA to implement the technology-neutral program, in cooperation with non-federal entities. The technology-neutral program is open to all methods of producing Mo-99 without using HEU.
More information will be added as it becomes available.
You can contact John Huotari, owner and publisher of Oak Ridge Today, at (865) 951-9692 or john.huotari@oakridgetoday.com.
Most news stories on Oak Ridge Today are free, brought to you by Oak Ridge Today with help from our advertisers, sponsors, and subscribers. This is a free story. Thank you to our advertisers, sponsors, and subscribers. You can see what we cover here.
Do you appreciate this story or our work in general? If so, please consider a monthly subscription to Oak Ridge Today. See our Subscribe page here. Thank you for reading Oak Ridge Today.
Copyright 2019 Oak Ridge Today. All rights reserved. This material may not be published, broadcast, rewritten, or redistributed.
Leave a Reply